The Specific Gravity (SG) of a substance is a measure of its density. It is expressed as the ratio of the weight of a given volume of the substance compared to the weight of an equal volume of a reference substance – usually water [1] . An alternative term sometimes used is: WEIGHT PER UNIT VOLUME. The reference substance (water) is given an SG of 1. In the International System of units (SI) the weight of water is defined in terms of its of volume: thus 1 litre of water weighs 1 kilogram and 1 cubic meter (1000 litres) of water weighs 1 tonne (1000 kg). Thus the weight in tonnes of any substance whose volume is expressed in cubic meters can be simply calculated by multiplying that volume by its SG. For example: a cubic meter of a rock with an SG of 2.5 weighs 2.5 tonnes.
Different rocks have different Specific Gravities. In broad terms, quartzo-feldspathic (felsic) igneous rocks (granite, rhyolite) have SGs in the range of 2.4 – 2.8. Basic igneous rocks such as basalt or dolerite (diabase) have SGs from 2.8 – 3.0 whilst the SGs of ultramafic igneous rocks (pyroxenite, peridotite, komatiite etc..) range from 3.0 – 3.5. Sedimentary rocks have a wide range of SGs depending on their composition, porosity and degree of lithification, but generally are in the range of 2.4 – 2.8.
Sulphide minerals, which can make up significant percentages (often to 100%) of some metallic ore bodies, have much higher SGs than their unmineralised host rocks. Thus:
Galena 7.2 – 7.6 Sphalerite and chalcopyrite 4 .1 – 4.3
Pyrite and pyrhotite 4.9 – 5.1 Pentlandite 4.6 – 5.0
Of course, some ore bodies might have an SG that is less than their surrounding rocks. An example would be a gold bearing quartz vein (SG 2.6) in a basalt host. Even although gold has an SG of over 19, there is seldom enough of it present in a quartz vein to affect the overall density of the rock, and gold and quartz must be mined together.
Mine planners need to know the weight of the rocks they are going to extract and this means they need to know the exact Specific Gravity of each of the major rock types within their mine or proposed mine. When dealing with hundreds of thousands of cubic metres of rock, even a difference of 0.1 in SG can make a very large difference to the final calculated weight. Ore bodies that consist of metallic sulphides may have a range of specific gravities depending on the amount and type of sulphide present with low-grade ore usually at a lower specific gravity than high grade ore. This needs to be taken into account when calculating the tonnes of ore present in a mine.
Accurate measurements of SGs are needed. To get these figures representative samples of the rocks, usually small pieces of drill core 10-20cms long, are taken. For each major rock type at least 30 such samples from different parts of the mine environment, are measured for their SG and the results averaged. Such measurements are generally undertaken when mine exploration reaches advanced feasibility or pre-feasibility stage and the need arises for calculation of tonnes of ore and waste.
In addition to mine planning, knowledge of the accurate SG of various rock types in an area is needed by geophysicists seeking to analyse gravity measurements.
Outlining practical techniques for field measurement of SG on drill core on small rock hand specimens is the purpose of this blog post. There are two techniques:
1. The first technique is to use a diamond saw to cut a square-ended section of core about 10-15cm long. The known length and diameter of this regular cylinder allows its volume to be easily calculated. It can then be weighed using a sensitive balance. By comparing volume (in cubic cms) to weight (in grams) the SG can be calculated. However, this process is time consuming, and drill core is often fractured into irregular pieces, which makes cutting regular cylinders impracticable. Rock specimens collected from outcrop are almost always irregularly shaped. There is a better way.
2. Archimedes Principle states that the upwards force on a body immersed in fluid is equal to the weight of the fluid that the body displaces[2]. From this we are able to define SG in terms which make it easy to measure : SG = Weight of Sample divided by (Weight of Sample in Air minus Weight of Sample in Water)
Or: SG = W(air) / W(air) – W(water)
The sequence of required measurement is illustrated in the diagram below. 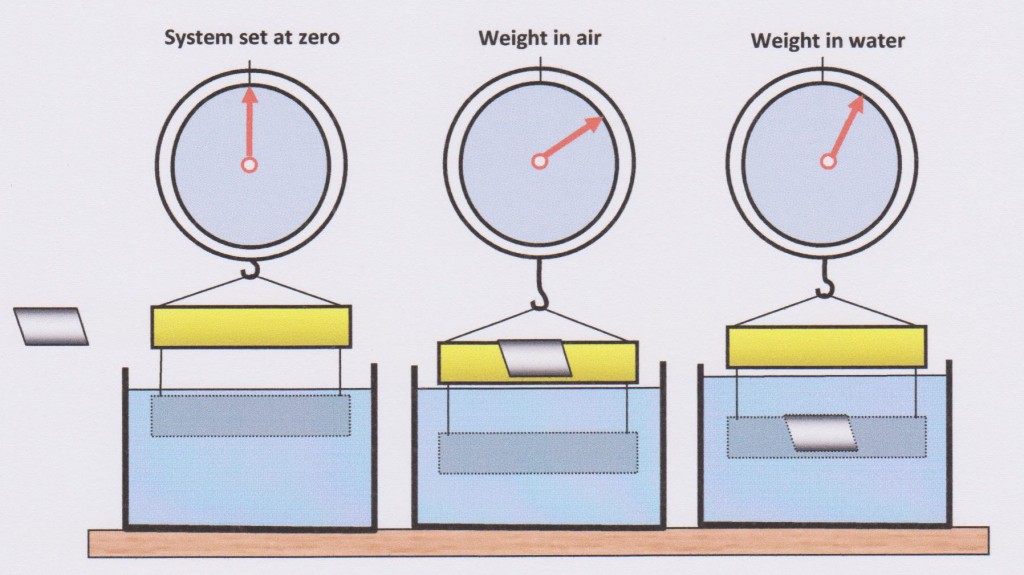
Some practical tips;
- The sample chosen should be as near representative of the rock unit as can be found.
- The specimen should be a solid homogenous rock, of uniform composition and free from surface mud, grease, cracks and vesicles.
- Special problems arise where the specimen is porous, with visible holes or vesicles, and has been recovered from above the water table so that in its original state these holes were filled with air. This can arise when drilling the upper levels of some epithermal systems. The best way to measure the SG of such rocks is to cut a regular cylinder (if that is possible) and calculate its volume using Method0d 1, above. There is no really satisfactory way to measure the SG of such rocks using water immersion because water will penetrate the specimen, driving out air, and increasing its measured SG. But if you have to use this method, two techniques have been employed. The first method is to tightly wrap the rock specimen in plastic cling film. However, in practise, it can be difficult to exclude bubbles of air being entrapped on the surface of the core beneath the film (this would increase the volume and so give a lower SG). Another solution to the problem is to spray the specimen with a thin film of hot wax before immersion. However, while this might seal off small holes, large holes will still be open to water ingress on immersion.
- An accurate scale capable of weighing to +/- 1 gram is needed. The use of an electronic laboratory balance is indicated.
- When the specimen is submersed in water, shake and tap it for a few seconds to remove any air bubbles that might cling to it (this is especially important if the surface of the specimen if rough and indented) before weighing.
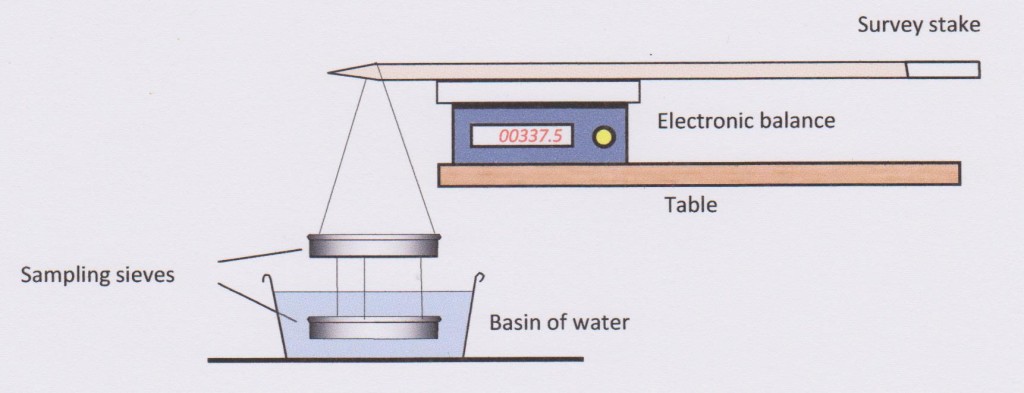
- The set up illustrated below has proved to be a practical arrangement for measuring the SG of specimens in the field.
[1] Or, if you want to be really accurate, the weight of water in its most dense form at 4° C.
[2] Archimedes of Syracuse (287-212BC). According to myth, Archimedes discovered this principle while taking a bath and pondering the problem of determining the density of a crown (maybe pure gold, maybe not) which the local King had given him for testing. Pleased with his neat solution, Archimedes sprang from his bath and ran naked through the streets of Syracuse crying Eureka (I have it!). One of the greatest scientists of antiquity, Archimedes deserves better than this apocryphal story, but it is a gift for cartoonists nevertheless…..